Stay updated
Receive the latest news and information about achondroplasia, including when new content such as patient resources are added to the website.

Achondroplasia is the most common type of skeletal dysplasia and is characterized by impaired endochondral bone growth1

Impaired bone growth impacts individuals physically, functionally, and psychosocially

Sort and expand the latest insights into achondroplasia diagnosis, management, treatment, and more according to developmental stage with this interactive tool.
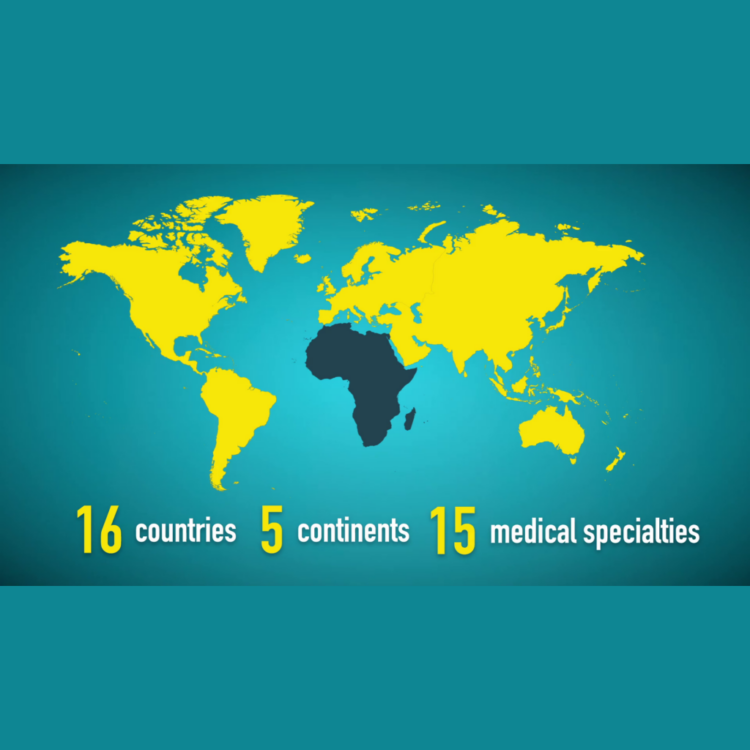
The International Consensus Statement on the diagnosis, multidisciplinary management and lifelong care of individuals with achondroplasia
Parents, caregivers, and individuals with achondroplasia may need a strong medical community from day one1

Primary care providers can help to coordinate a multidisciplinary approach to care

Everything you need to deepen your achondroplasia knowledge
Receive the latest news and information about achondroplasia, including when new content such as patient resources are added to the website.
Are you a Healthcare Professional practicing in the U.S.?